Regionaalhaigla ja Kliinikumi arstide ning Tartu Ülikooli teadlaste uurimistöö tulemusena ilmus 2019. aasta detsembris teadusajakirjas “Injury“ teadusartikkel “The role of elevated high-sensitivity cardiac troponin on outcomes following severe blunt chest trauma“, millega saab lähemalt tutvuda siin.
The role of elevated high-sensitivity cardiac troponin on outcomes following severe blunt chest trauma
Triinu Keskpaik, Joel Starkopf, Ülle Kirsimägi, Vladislav Mihnovitša, Andrus Lomp, Eeva-Maarja Raamat, Sten Saar, Peep Talving
Abstract
Background: Blunt cardiac injuries (BCI) result in poor outcomes following chest trauma. Admission ECG and troponin levels are frequently obtained in patients with suspected BCI, nevertheless, the prognostic value of cardiac troponins remains controversial. The purpose of the current study was to review the prognostic value of elevated high-sensitivity cardiac troponin T (hs-cTnT) in patients with severe blunt chest injuries. We hypothesized that elevated hs-cTnT result in poor outcomes in this subgroup of severe trauma patients.
Methods: After IRB approval, all consecutive patients with Injury Severity Score (ISS) > 15 and chest Abbreviated Injury Scale (AIS) score ≥3 admitted to the major trauma centers between 1/2015 and 6/2017 were retrospectively reviewed. Primary outcomes were in-hospital and one-year mortality. Secondary outcomes included ventilator days and Glasgow Outcome Scale (GOS) score at hospital discharge.
Results: Overall, 147 patients were included. Mean age was 49.0 (19.1) years and 75% were male. Serum troponin levels on admission were accrued in 82 (56%) patients with elevated and normal hs-cTnT levels found in 54 (66%) and in 28 (34%) patients, respectively. Elevated hs-cTnT group had significantly higher ISS and lactate level, and lower systolic blood pressure on admission. In-hospital mortality was significantly higher in patients with elevated hs-cTnT levels compared to patients with normal hs-cTnT levels (26% vs. 4%, p = 0.02). Hs-cTnT level > 14 ng/L was significantly associated with extended ventilator days and lower GOS score at hospital discharge.
Conclusion: Blunt chest trauma victims with elevated hs-cTnT levels experience significantly poorer adjusted outcomes compared to patients with normal levels. Compliance with EAST practice management guidelines following severe blunt chest trauma was not fully complied in our study cohort that warrants prospective performance improvement measures.
Background
Blunt cardiac injury (BCI) may result in a variety of lesions including dysrhythmias,myocardial contusions, pericardial rupture,
septal injuries, valvular and chorda tendinea lesions, myocardial rupture, and commotio cordis [1]. In a recent autopsy study, 8% of all blunt trauma fatalities were related to cardiac injuries resulting most frequently in right ventricular rupture [2]. In a prospective pilot study, 28% of the major trauma patients (ISS > 12) with chest injury and elevated cardiac troponin I (cTnI) levels had abnormal cardiac magnetic resonance imaging including myocardial edema, regional wall motion abnormality and myocardial hemorrhage [3].
The diagnostic criteria for BCI are not well defined [4]. The reported incidence of BCI ranges between 13% and 50% depending on criteria used for the diagnosis [5–9]. BCI clinical spectrum varies from asymptomatic ECG changes to life-threatening cardiac complications. It has been noted that severe complications including arrhythmias requiring treatment, hypotension, cardiogenic shock or hemopericardium requiring pericardiocentesis occur most frequently within 24 h of admission [5].
The Eastern Association for the Surgery of Trauma (EAST) guidelines recommend admission electrocardiogram (ECG) and cTnI screening in all patients with suspected BCI combined with echocardiography, computed tomography or magnetic resonance imagining, on demand [10]. However, the EAST recommendations are exclusively based on studies utilizing conventional cardiac troponin assays.
Cardiac troponin I (cTnI) and T (cTnT) are components of the contractile apparatus of myocardial cells expressed almost exclusively in the myocardium, thus, being specific biomarkers for diagnosis or exclusion of myocardial infarction [11]. Minimal concentrations of cTn can be detected in a blood serum using recently introduced high-sensitive troponin (hs-cTn) assys, thus, a higher proportion of patients have cTn value higher than 99th percentile. Although cardiac troponins are specific for myocardial damage, the exact underlying mechanism is not always clear. Non-acute coronary syndrome-associated elevation of hs-cTn levels including trauma is often observed in the emergency department (ED) [12]. The purpose of this investigation was to evaluate the predictive value of elevated hs-cTnT levels in patients with severe chest injuries after blunt trauma. We hypothesized that elevated hs-cTnT result in poor outcomes in this subgroup of severe trauma patients.
Methods
After IRB approval, all consecutive trauma admissions with In- jury Severity Score (ISS) > 15 and chest Abbreviated Injury Scale (AIS) score ≥3 to two national referral trauma facilities between 1/2015 and 6/2017 were retrospectively identified from electronic health record database. Both admissions from the scene of injury and interhospital transfers were included. Exclusion criteria were penetrating injuries, patients deceased in ED and cases with deficient medical records. The AIS C version 2005 Update 2008 was utilized to calculate ISS scores [13] . For confounder analysis, a respective group of all consecutive major trauma (ISS > 15) admis- sions without severe chest injury (thorax AIS < 3) was identified.
Data collection included demographics, previous cardiovascular disease, Charlson Comorbidity Index (CCI), vital signs and laboratory markers on admission, Glasgow Coma Scale (GCS) score, ISS and AIS scores. Diagnostics and treatment procedures performed within 72 h from admission including electrocardiograms (ECG), focused assessments with sonography for trauma (FAST), computed tomography (CT), transthoracic or transesophageal echocardiography (TTE, TEE), vasopressors, inotropes and antiarrhythmic drug use, synchronized electrical cardioversions, blood components transfusion, and pericardiocentesis were accrued.
The diagnosis of BCI included an elevated hs-cTnT level with abnormal ECG or echocardiography findings. Hs-cTnT was measured with an electrochemiluminescence immunoassay method (Cobas e601; Roche Diagnostics, Indianapolis, IN, USA) and was defined abnormal when values exceeded 99th percentile (> 14 ng/L). The ECG changes indicating a cardiac injury were arrhythmias (other than sinus rhythm), a pattern of bundle branch block, a pro- longed QT-interval, Q-wave formation, ST-segment depression or elevation more than 1 mm and a flat or inverted T-waves or both, in two or more leads [5, 14–15]. The echocardiographic changes referring to cardiac injury were pericardial effusion, regional wall motion abnormality, acute valvular dysfunction, right or left ventricle dilatation, ventricular septal rupture, and intracardiac thrombus [4]. Clinically significant BCI was diagnosed if one of the fol- lowing interventions were required: vasoactive, inotropic, antiarrhythmic agent used, pericardiocentesis or surgical management of cardiac injury required. Primary outcomes were in-hospital and one-year mortality. Secondary outcomes included length of intensive care unit (ICU) stay, ventilator days, antiarrhythmic intervention, vasopressor and inotropic agent use, and GOS at hospital discharge. Follow-up Kaplan–Meier survival analysis was con- ducted in 9/2018. Mortality data was collected from Estonian National Causes of Death Registry.
All statistical analyses were performed with Statistica version 13 (TIBCO Software Inc., CA, USA). Means and standard deviations (SD) are reported for normally distributed data. For not normally distributed data, medians and interquartile ranges (IQR) were deployed. Comparisons between groups were performed using the Student‘s t -test (two-sided) for normally distributed variables and Mann–Whitney U test for not normally distributed variables. For categorical variables Fisher’s exact test was deployed and p -values were calculated as double the exact one-tailed probability [16] . A survival analysis based on the Kaplan–Meier curves was per- formed. The difference between two Kaplan–Meier survival curves was calculated using the log-rank test. Multivariable Cox proportional hazard model was deployed to determine the relationship between patient one-year mortality and demographics, vital signs, and laboratory data. All variables different at a p < 0.2 level in a univariate analysis were subjected to multivariable analysis. For all statistical tests a p -value < 0.05 was considered significant.
Results
During the 30-month study period, 171 patients with ISS ≥15 and chest AIS ≥3 were identified. Patients with penetrating injuries (n = 13), patients deceased in ED (n = 8) and cases with deficient medical information (n = 3) were excluded with 147 cases included for the final analysis (Fig. 1). Overall, 137 patients were admitted from the scene and 10 patients were transferred from outside hospitals.
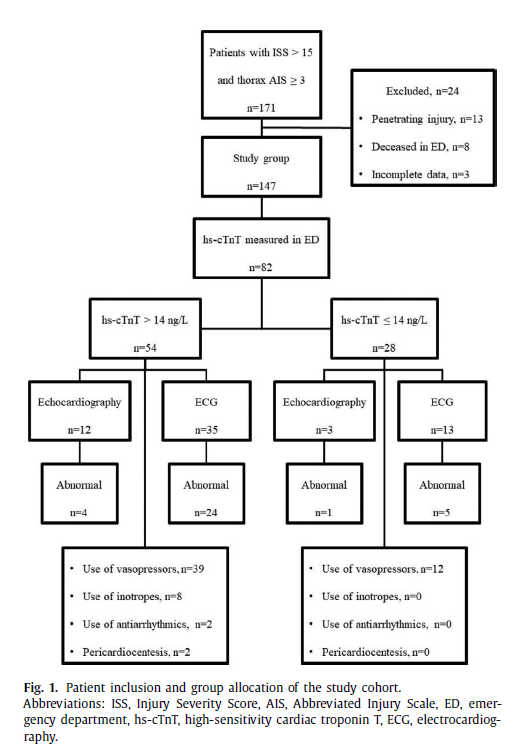
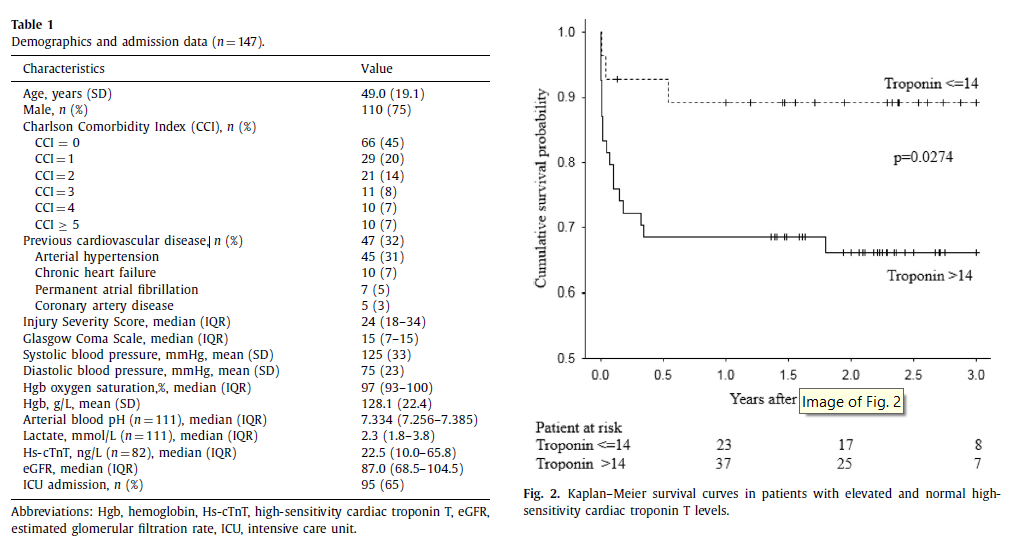
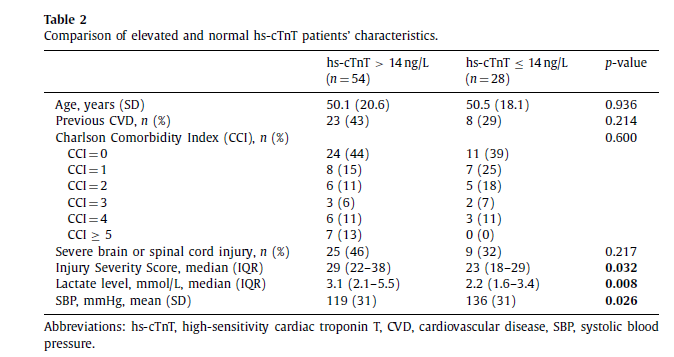
Table 1 shows demographics and admission data of patients with thorax AIS ≥3. Majority of patients were male with a mean age of 49.0 years. Median ISS was 24. Thirty-two percent (47/147) of patients had a history of previous cardiovascular disease with arterial hypertension being the most frequent comorbidity.
Serum troponin levels on admission were obtained in 82 (56%) patients with elevated hs-cTnT level found in 66% (54/82). Overall, median hs-cTnT level was 22.5 ng/L (IQR 10.0–65.8), however, median troponin level in the subgroup with elevated hs-cTnT ( > 14 ng/L) was 41.7 ng/L (IQR 23.0–83.0 ng/L). Admission ECG was performed in 73 (50%) patients with abnormal findings in 34 cases (47%). Echocardiography was performed in 24 (16%) patients resulting in abnormal findings in 9 patients (38%). Six patients were found to have pericardial effusion with three requiring urgent thoracotomy for cardiac injury repair (two patients with right atrial rupture and one patient with right pulmonary vein rupture). Overall in-hospital mortality was 17% (25/147).
For further analysis, the thorax AIS ≥3 patients were divided into two subgroups based on their troponin values ( n = 82): elevated troponin group (hs-cTnT > 14 ng/L) and normal troponin group (hs-cTnT ≤14 ng/L), respectively ( Fig. 1 ). Comparison be- tween the groups is depicted in the Table 2. Eighteen patients had clinically significant BCI per the study definition. Patients with elevated hs-cTnT had significantly higher ISS (29 vs. 23, p = 0.032), lower systolic blood pressure on admission (119 mmHg vs. 136 mmHg, p = 0.026) and higher arterial blood lactate level (3.1 mmol/L vs. 2.2 mmol/L, p = 0.008) compared to the subgroup with normal hs-cTnT.
In-hospital mortality in the elevated troponin group and in the group with normal troponin level was 26% (14/54) and 4% (1/28), respectively (p = 0.02). One-year mortality was 32% (17/54) in the elevated troponin group and 11% (3/28) in the group with normal troponin level ( p = 0.063). Kaplan–Meier survival estimates are shown in Fig. 2 . Patients with elevated troponin had lower GOS at hospital discharge (median GOS 3 [IQR 1–4] vs. 4 [IQR 4–5], p = 0.001), longer ventilator days (median ventilator days 4.0 [IQR 2.0–13.0] vs. 0.0 [IQR 0.0–7.0], p = 0.005) and trend towards longer stay in the ICU (median ICU days 7.0 [IQR 3.0–18.0] vs. 4.5 [0.0–11.5], p = 0.051) compared to the normal troponin group.
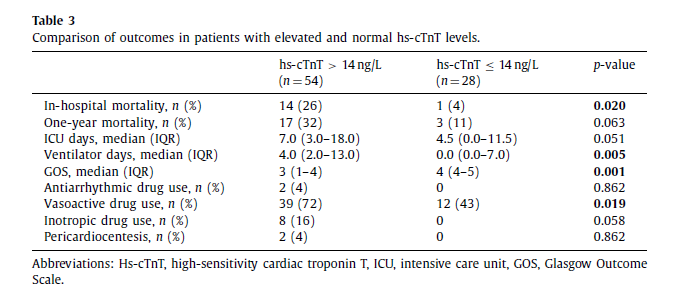
Vasopressor agent support was required in 39 (72%) and 12 (43%) patients in the elevated troponin group and normal troponin level group, respectively ( p = 0.019). Inotropic and antiarrhytmic agents were utilized only in elevated troponin group with no statistical significance compared to the group with normal troponin (Table 3).
Univariate analysis on one-year mortality included gender, age (< 60 years vs. ≥60 years), previous cardiovascular disease history, CCI (< 2 vs. ≥2), arterialblood lactatelevel(< 2 mmol/L vs. ≥2 mmol/L), troponin level ( ≤14 ng/L vs. > 14 ng/L), ISS (< 25 vs. ≥25), GCS ( < 8 vs. ≥8), mean arterial pressure (MAP) and hemoglobin level. The univariate analysis revealed possible relationship with age ( p = 0.007), CCI (p = 0.001), troponin level (p = 0.055), GCS (p = 0.004), MAP (p = 0.063) and hemoglobin level ( p = 0.035) (Supplemental Table 1). Independent risk factors for one-year mortality on multivariable Cox proportional hazard model were MAP on admission (hazard ratio 0.98, 95% CI 0.96–1.00, p = 0.03), CCI ≥2 (hazard ratio 7.63, 95% CI 2.66–21.95, p < 0.001) and GCS < 8 (hazard ratio 3.74, 95% CI 1.47–9.49, p = 0.005) (Supplemental Table 2).
In confounder analysis we identified a total of 136 patients with thorax AIS < 3 and 118 patients were eligible for the final analysis. Serum troponin levels on admission were obtained in 50 patients. Comparison of thorax AIS < 3 and thorax AIS ≥3 groups revealed that patients were similar in age, sex, CCI and previous cardiovascular disease history. Also, two groups did not differ per their admission hemoglobin levels or systolic blood pressures. The thorax AIS ≥3 group had significantly higher troponin level [median hs-cTnT 22.5 ng/L (IQR 10.0–65.8) vs. 18.0 ng/L (IQR 5.0–20.0), p < 0.001], lower ISS [median ISS 24 (IQR 18–34) vs. 25 (IQR 17–25), p = 0.003], higher GCS score [median GCS score 15 (IQR 7–15) vs. 12 (IQR 6–15), p = 0.002], higher arterial blood lactate level [median lactate level 2.3 mmol/L (IQR 1.8–3.8) vs. 2.1 mmol/L (1.4–3.2), p = 0.011], lower hemoglobin oxygen saturation [median SpO 2 97% (IQR 93–100) vs. 98% (96–100), p < 0.001], and lower estimated glomerular filtration rate [median eGFR 87.0 (IQR 68.5–104.5) vs. 103.0 (IQR 84.0–114.0), p < 0.001] compared to the thorax AIS < 3 group. Two groups had similar in-hospital mortality, one-year mortality and GOS at hospital discharge. Patients in thorax AIS ≥3 group had longer ventilator days compared to thorax AIS < 3 group [median ventilator days 3.5 (IQR 1.0–10.5) vs. 2.0 (IQR 0.0–5.0), respectively, p = 0.041].
Discussion
Blunt cardiac injury comprises a wide spectrum of conductive and structural cardiac lesions, however, the gold standard diagnostic tests are still a subject of a lively debate. According to the Advanced Trauma Life Support® guidelines, patients with a BCI diagnosed by conduction abnormalities on ECG are at risk for sudden dysrhythmias and should be monitored for the first 24 h [17] . Per the recent EAST guidelines, all patients with significant blunt trauma to anterior chest are recommended to be screened for BCI with ECG and troponin levels [10] . All previous reports and guide- lines have based their outcomes and recommendations on non- high-sensitivity troponin levels. The current study, however, investigated outcomes based on the hs-cTnT.
Elevated troponin levels are associated with many factors including age, gender, renal insufficiency, heart failure, and trauma. Harvell et al. demonstrated in a cross-sectional cohort study of random subset of patients with elevated troponins that the etiology for most (79%) initial elevated cTn levels was related to non-acute coronary syndromes (ACS). Non-ACS causes were mostly cardiovascular, infections, renal, and hypertension related. Nevertheless, the median initial cTn and peak cTn levels were higher in ACS related cases compared to the non-ACS related group [18] . In a cross-sectional analysis of emergency room patients without ACS, 52% had an initial hs-cTnT level exceeding the normal range. The most frequently encountered mechanism was renal insufficiency at 57% followed by cerebral ischemia (19%), trauma (15%) and cardiac failure (8%) [12] . Gore et al. observed that over 10% of male patients between 65 and 74 years without previous cardiovascular disease had hs-cTnT values above the 99th percentile [19] . Also, reduced eGFR is reported to independently and negatively being related to detectable hs-cTnT [20] . In our study, over 60% of patients had hs-cTnT levels exceeding the 99th percentile. However, our results did not show any significant difference in age, previous cardiovascular disease history, CCI and concomitant severe brain or spinal cord injury between our study groups. Majority of our study patients had mild or no renal insufficiency based on the admission eGFR value (median eGFR 87.0, IQR 68.5–104.5).
We report a high number of cases with severe traumatic brain injury (TBI) in the elevated hs-cTnT group ( Table 2 ). In previous studies, cardiac dysfunction and troponin release have been demonstrated in nontraumatic cerebrovascular events (predominantly subarachnoid hemorrhage) and also in TBI victims. The underlying mechanism is described in association with catecholamine-induced cardiac-toxicity resulting in contraction- band necrosis [21]. Salim et al. noted in their retrospective study that elevated cTn was documented following severe blunt TBI (head AIS ≥3 to the exclusion of AIS ≥3 for injuries of other anatomic regions) in almost 30% of patients. The authors concluded that the level of cTn correlates with severity of TBI and that elevated peak-troponin was an independent predictor of mortality in severe TBI victims with an odds ratio of 8.5 (95% CI 3.46–22.15, p < 0.0 0 01) [22] . In our study, from the total of 82 severely injured patients, 34 had a concomitant TBI. The incidence of TBI did not differ between the groups if divided according to their hs-cTnT levels. Also, the patients in thorax AIS < 3 group presented with lower GCS, but had significantly lower admission hs-cTnT levels.
Approximately half of patients with blunt chest trauma demonstrate ECG changes, however, aberrant ECG findings only do not indicate a significant BCI [5, 6]. In our study, an admission ECG was performed only in half of the patients with 47% (34/73) suffering conduction abnormalities, ST-segment/T-wave changes or arrhythmias. Based on our data, ECG remains as a valid screening tool for BCI.
Another diagnostic method for BCI is echocardiography, used in patients with hemodynamic instability or a persistent new dysrhythmia, but not as a primary screening modality [10] . Velma- hos et al. found that echocardiography revealed abnormalities in half of the patients with significant BCIs [6] . Similar findings were reported by Salim and co-authors [5]. Likewise, Boeken et al. observed in a retrospective analysis of blunt chest trauma victims that 7 patients of 17 had abnormality by echocardiography [8]. In our study, echocardiography was performed in 24 patients with suggestive changes of cardiac injury noted in 9 patients (38%) and 3 patients (13%) had a structural lesion requiring surgical repair.
The prognostic value of cTn is controversial per the previous reports. It has been alluded in many prospective studies that sensitivity and negative predictive value to rule out clinically significant BCI in settings of normal initial ECG and normal troponin levels is 100% [5 , 6]. However, Bertinchant et al. concluded that cTnT and cTnI measurements did not improve sensitivity of the diagnosis of myocardial contusion and cardiac troponins increase had a poor relationship with clinical outcomes in hemodynamically stable blunt chest trauma patients [14]. In a prospective study by Burrell et al., elevated troponin levels were found to be highly sensitive (100%) but not specific (21%) in predicting major adverse cardiac events, including inotropic support requirement, atrial fibrillation, ventricular tachycardia and tricuspid valve rupture requiring surgical replacement in severe trauma victims (ISS > 12) [3]. In a retrospective analysis by Joseph et al., cTnI, hypotension and elevated lactate were independent predictors of mortality in BCI patients [23] . Martin et al. concluded in their retrospective analysis that increased serum cTnI is related to the degree of overall injury and physiologic stress not mechanical chest trauma. Nevertheless, these authors noted that elevated serum cTnI was an independent predictor of mortality in trauma ICU patients [24] . In our cohort, 18 patients were found to have clinically significant BCI per the study definition. Comparison of normal and elevated hs-cTnT groups revealed that patients with elevated troponin level had higher ISS, higher admission lactate level and lower systolic blood pressure on admission. Additionally, more vasopressor support was required in the elevated troponin group and hs-cTnT > 14 ng/L was associated with increased in-hospital mortality, longer ventilator days and worse functional outcome compared to those with normal hs-cTnT levels. There was likewise a trend towards longer stay in the ICU and higher requirement of inotropic support in the elevated troponin group.
Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study investigators demonstrated in a large international prospective study the prognostic significance of a troponin elevation after noncardiac surgery. Elevated postoperative cTn was associated with increased 30-day mortality even in the absence of ischemic features. The term myocardial injury after noncardiac surgery (MINS) represents a prognostically relevant myocardial injury caused by ischemia occurring during or within 30 days after noncardiac surgery. Perioperative troponin monitoring is essential for establishing the diagnosis of MINS as only minority of patients experience an ischemic symptom and ischemic ECG findings [25 , 26]. Our data includes admission troponin levels only precluding assessment of the perioperative myocardial injury in our study cohort.
The current study has inherited limitations due to its retrospective design. Secondly, hs-cTnT values were not obtained routinely in our study cohort. Thirdly, BCI was defined as a combination of elevated hs-cTnT and ECG or echocardiography findings with BCI considered to be clinically important when the condition required specific interventions. However, ECG was performed only in 65% (35/54) and echocardiography in 22% (12/54) of patients with elevated hs-cTnT translating into potential underestimation of clinically significant BCI prevalence. Hs-cTnT samples were obtained on admission, thus, the exact time from trauma to blood sampling remains undetermined. Also, 10 patients were transferred from other hospitals (troponin was measured in six of them) and this may have an impact on their admission data. Finally, our study is lacking data for serial hs-cTnT measurements, thus, the trend of hs-cTnT values are not reported. Nevertheless, to the best of our knowledge this is the very first study investigating the utility of hs-cTnT following blunt chest injuries.
Despite the limitations, this study indicates that severely injured blunt trauma victims suffering a concomitant severe chest trauma associated with elevated hs-cTnT, experience poorer out- comes compared to patients with normal hs-cTnT level. Patients with elevated hs-cTnT have higher mortality and higher need for vasopressor support. As noted in previous studies, cardiac troponin could be a general marker of injury severity, however, in this study, we emphasize chest AIS ≥3 translating into an effect of BCI on out- comes. We suggest routine troponin measurement to screen for BCI because elevated troponin might indicate the need for ICU admission, closer monitoring and further diagnostic tests performance for these patients.
Conclusion
Blunt chest trauma victims with elevated hs-cTnT levels experience significantly poorer adjusted outcomes compared to those with normal hs-cTnT levels. Compliance with EAST practice management guidelines in severe blunt chest trauma was not fully complied in our study cohort and warrants performance improvement measures.
CRediT authorship contribution statement
Triinu Keskpaik: Investigation, Data curation, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Joel Starkopf: Investigation, Methodology, Data curation, Writing - original draft, Writing - review & editing. Ülle Kirsimägi: Methodology, Data curation, Writing - original draft, Writing - review & editing. Vladislav Mihnovitš: Methodology, Data curation, Writing - original draft, Writing - review & editing. Andrus Lomp: Methodology, Data curation, Writing - original draft, Writing - review & editing. Eeva-Maarja Raamat: Methodology, Data curation, Writing - review & editing. Sten Saar: Methodology, Writing - review & editing. Peep Talving: Investigation, Methodology, Data curation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
Funding
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.injury.2019.12.037.

